By Avigayil Kadesh
Within the next decade, most surgical procedures will not require a single stroke of a scalpel, predicts Kobi Vortman, president and founder of InSightec , an Israeli firm whose unique ExAblate technology for the “operating room of the future” will replace standard operations with non-invasive outpatient procedures.
The ExAblate MRI-guided high-intensity ultrasound technology destroys targets (such as tumors) deep inside the body without incisions, while the patient lies in an MRI.
The procedure is controlled and monitored in real time, allowing the doctor operating the system to change treatment parameters on the fly, as needed. TIME magazine chose focused ultrasound technology as one of 50 best inventions of 2011.
Meanwhile, two of the company’s three product lines – for women’s health and oncology – have already received regulatory approvals around the world including Europe’s CE Mark and the US Food and Drug Administration (FDA). The third product line, the ExAblate Neuro, recently received the CE Mark for the treatment of neurological disorders.
ExAblate O.R., a non-invasive operating room, incorporates interchangeable “cradles” for each application that are interfaced with the common therapy table.
“We started with uterine fibroids, which afflict a quarter of all women sometime in their lives and usually is treated with a hysterectomy,” says Vortman.
In January 2013, patient recruitment got underway for a comparative study at the Mayo Clinic and at Duke University to determine how ExAblate stacks up to the usual procedure, uterine artery embolization. “They’ve treated 15 patients so far,” Vortman said in late February.
The two-venue study is to include about 200 patients over three years. “We believe for patients looking for alternative treatment to preserve the uterus, this is effective and safe,” he says.
The technology has already been successfully used to destroy fibroids in more than 9,000 patients in the United States, Europe and Asia. The comparative data from the current study is for medical insurance reimbursement purposes.
ExAblate’s use in alleviating pain from metastatic bone tumors completed a pivotal trial last year that led to FDA approval for this application in October 2012. The next step will be a randomized clinical trial to compare radiation therapy – currently the gold standard for this condition – with ExAblate as a first line of defense.
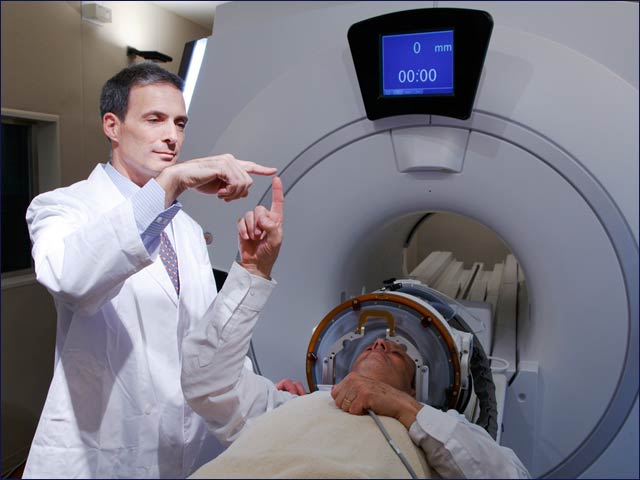
Patients are monitored in real time
during the non-invasive procedure
Vortman explains that the first trial focused on patients who had failed to improve with radiation or who had declined radiation treatment.
“We want to expand our study to treat newly diagnosed patients. That will be international and randomized,” says Vortman, who has degrees in electrical engineering and electro-optics from the Technion-Israel Institute of Technology. The trials will eventually include 10 to 15 sites in five to seven countries, including Israel.
New hope for brain disorders
At the same time, InSightec is moving forward with FDA-approved safety and effectiveness trials involving ExAblate Neuro for the non-invasive treatment of essential tremor, tremors related to Parkinson’s disease, and neuropathic pain.
The procedure, recently approved for use in European hospitals for these three applications, leaves the patient’s skull intact and requires neither electrode placement nor ionizing radiation.
Neurosurgeons and physicists at leading research hospitals in Switzerland, Korea, Tokyo, Canada and the United States are currently using ExAblate Neuro experimentally to treat essential tremor, a common movement disorder affecting millions of people worldwide.
More than one-third of patients with essential tremor become severely disabled and have difficulty performing everyday tasks like drinking, eating, dressing and writing. Those who do not respond to medication may consider surgical implantation of deep brain stimulating electrodes, or radiosurgery with ionizing radiation.
“Essential tremor is a relatively clear-cut disease with a single symptom,” explains Vortman. “With ExAblate Neuro, we found that at the end of a single treatment, patients suffering for years from this debilitating disease are back to themselves again.”
How long the effects last is not known yet because the 15 patients were treated at the University of Virginia only in the last year or two. Says Vortman, “For now, it looks very promising.”
The first Parkinson’s patients in a Phase III study are expected to be enrolled this year, with the hope of devising a full treatment protocol by the end of next year in order to receive FDA approval for this application.
In addition, says Vortman, “We are assessing the feasibility of treatment of epilepsy as well.”
Possible future applications for ExAblate are dissolving blood clots that cause stroke, and targeted drug delivery. Vortman says the system could be used for almost any surgery aside from lung tumors and heart valve repair.
The company, based in Tirat Carmel in the north with a second office in Or Yehuda near Tel Aviv, was founded in 1999 and has 125 employees. The company’s original R&D team all were Technion graduates like the founder Vortman.
InSightec is privately held by Elbit Imaging, General Electric, and MediTech Advisors. Last December, it completed a $30.9 million financing round. It has won several awards including The Wall Street Journal Technology Innovation Awards and the European Union's IST grand prize.